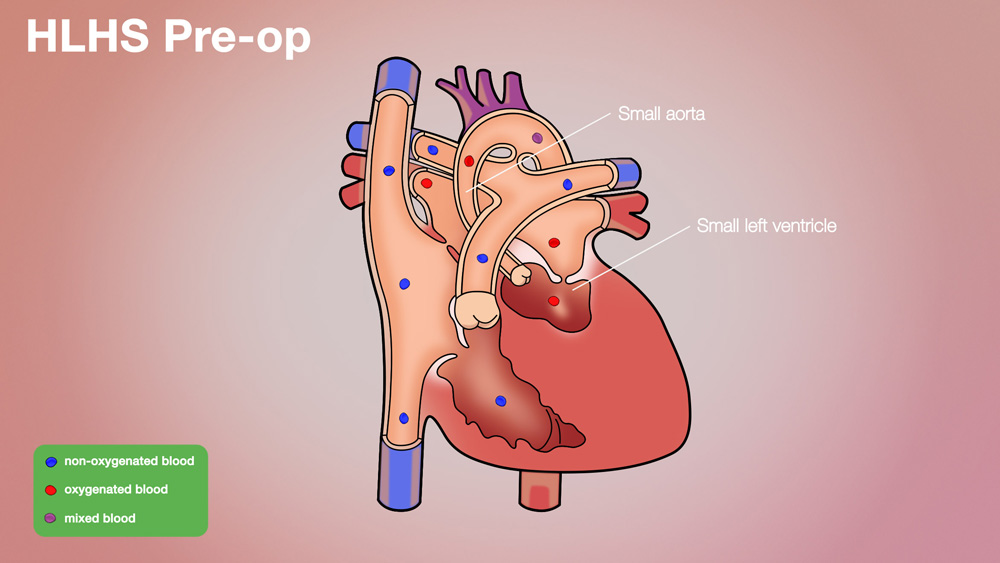
Typically, your child can be delivered without inducement and without a cesarean section (C-section), unless your obstetrician deems it necessary for other reasons.
A delivery plan will be developed carefully by your multidisciplinary team of affiliated physicians at The Fetal Center and the Children’s Heart Institute and will be discussed with you and your obstetrician. Your baby should be delivered at a tertiary care hospital that is well prepared to care for babies with congenital heart defects. There should be a neonatal intensive care unit with the capability to provide specialized care and pediatric cardiology and pediatric cardiovascular surgery services available at that institution.
Immediately following delivery, doctors will evaluate your baby carefully and, in most cases, transfer the newborn to a neonatal intensive care unit, where he or she will be stabilized and assisted with any breathing and blood pressure concerns. Medicine will be given to help stabilize your baby and support him or her until surgery can be performed.
The Fontan Palliation Sequence
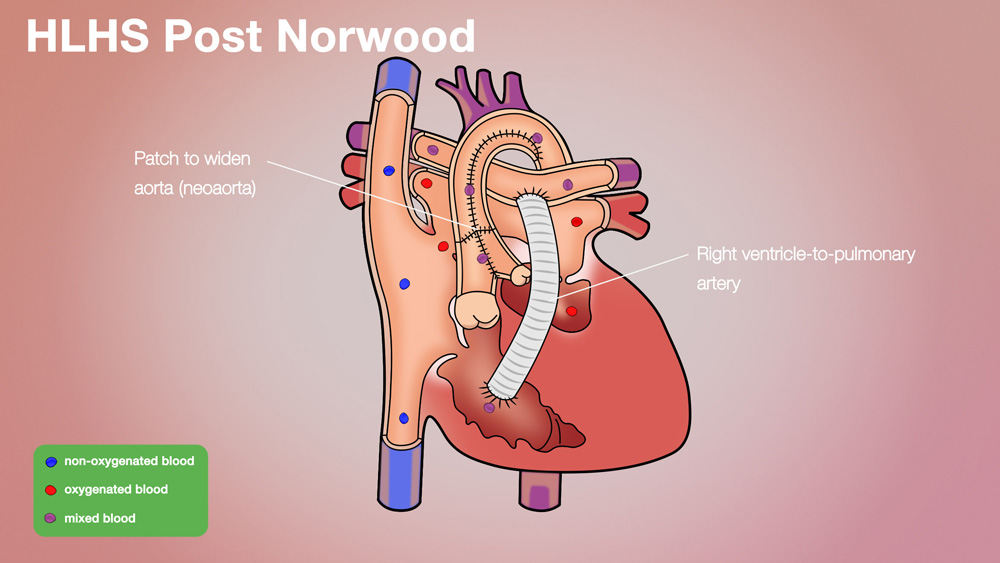
STAGE ONE | Norwood Procedure: The Norwood procedure is the first stage in a series of three open heart procedures to palliate HLHS. Typically, a Norwood procedure is done within the first two weeks of life and represents a high-risk, extensive operation. The principle behind the Norwood procedure is that the only good pumping chamber (the right ventricle) must pump blood to the body. There is no natural, permanent connection between the right ventricle and the body. Thus, the Norwood procedure involves dividing the blood vessel that takes blood from the right ventricle to the lungs and rerouting it to take blood to the body.
By dividing the blood vessel to the lungs, the blood supply to the lungs is taken away. Part of the Norwood procedure involves restoring some blood flow to the lungs. There are two methods generally used:
Modified Blalock-Taussig (MBT) shunt – a small tube from the main blood vessel to the body (aorta or branch of the aorta) that connects to the blood vessel to the lungs (pulmonary arteries)
Sano shunt - tube goes from the pumping chamber (right ventricle) to the blood vessel to the lungs (pulmonary arteries)
Both methods are accepted. The decision to use one versus the other is individualized based on the infant’s anatomy and the surgeon’s preference.
STAGES TWO & THREE: the blood which returns from the body is directly routed to the lungs without passing through the heart. (There is no ventricle pumping blood through the lungs as there is in a normal heart.)
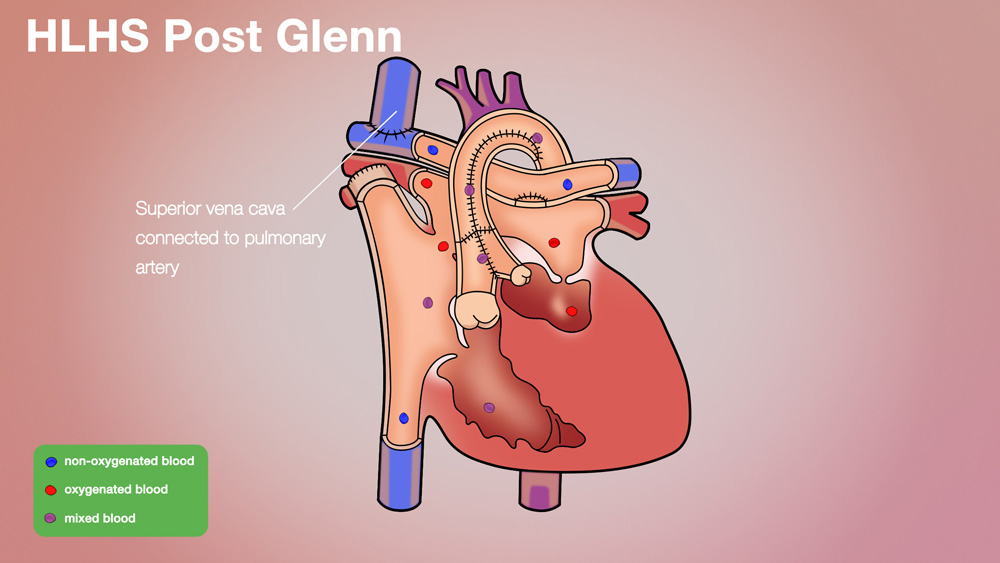
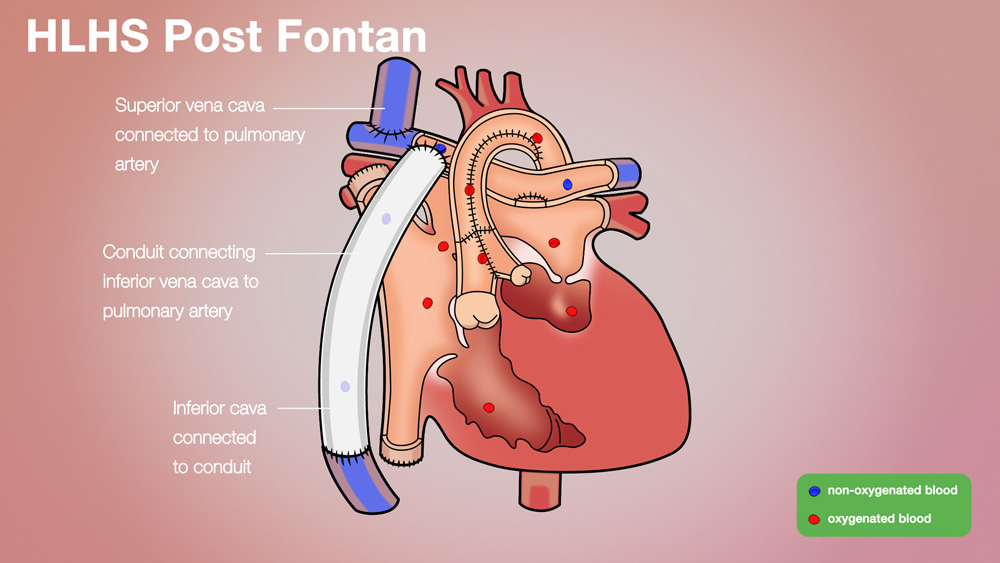
Bidirectional Glenn Procedure and Fontan Procedure:
Among doctors and nurses, a Fontan refers to the procedure where the two big veins that bring blood back from the body (one from the upper body and one from the lower body) are connected directly to the lungs. This is done at two separate operations. The upper vein is usually connected at about 6 months of age), and the lower vein is usually connected between 2 ½ and 5 years of age. The first operation is called a bidirectional Glenn procedure, named after Dr. Glenn, who first performed the procedure. The second operation is referred to as a Fontan procedure, named for Dr. Fontan.
In a bidirectional Glenn procedure (Glenn), the large vein which brings blood without oxygen back from the upper body is connected directly to the lungs. This blood is oxygenated by the lungs, and then is returned to the heart. The blood which comes back from the lower body goes directly to the heart itself, and then gets re-pumped to the body without going to the lungs. Since unoxygenated blood from the lower body gets mixed in the heart (with the oxygenated blood from the upper body), and then is pumped to the body, the blood that goes to the body is not fully oxygenated. The usual oxygen saturation for a child with a Glenn is around 80%. Children tolerate this level quite well, even though it is lower than a normal child’s oxygen saturation.
In a Fontan procedure, the large vein which carries unoxygenated blood to the heart form the lower body is connected directly to the artery that carries blood to the lungs. At the end of this operation, all of the unoxygentated blood that comes from the body goes to the lungs, and all of the blood that comes from the lungs goes to the body. The outcome is a normal blood flow pattern; however, the child’s heart is not structurally normal.
There are a number of reasons why the procedure is staged; that is, the upper vein is connected to the lungs at one operation, and the lower vein is connected at a different operation. Over time, this has shown to be a safer and better strategy for children, rather than doing the upper and lower veins during the same operation. Recovering from a Fontan can be very hard, and the heart has to be in the best shape possible in order for the Fontan to be successful.
A Glenn is not as hard to recover from as the complete Fontan, and a child whose heart is not in perfect shape will have a safer surgery with a Glenn than with a Fontan. A Glenn does not require additional work by the heart. Thus, when the Fontan surgery is performed, the heart is entering the operation in better condition.
Although the Fontan procedure is the best that can be offered for single ventricle patients at this time, it is far from perfect. With a Fontan, there is no ventricle to pump blood through the lungs. Only the “pressure” within the veins drives blood through the lungs. If there is any significant resistance to flow through the lungs, a Fontan will not work.