There are several types or variations of spina bifida. These variations depend on how the neural tube defect develops, what is protruding through the open neural tube, and what is covering the spina bifida abnormality. These variations include:
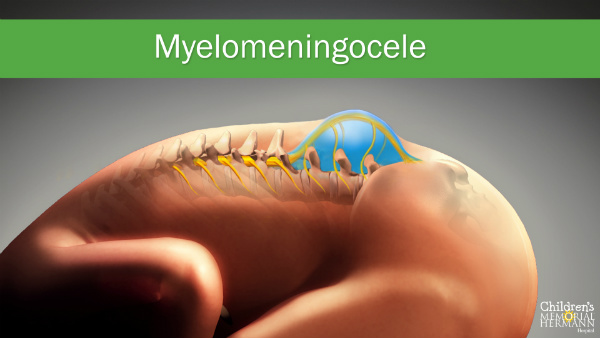 Myelomeningocele (MMC)
is the most common and most severe form of spina bifida. With myelomeningocele, a sac of fluid that holds part of the spinal cord and nerve tissue protrudes through an opening in the baby’s back. This damages the spinal cord and nerves through exposing them to the amniotic fluid. MMC can result in partial or complete paralysis of the body below the level of the spinal opening, an inability to walk, and/or bladder and bowel dysfunction.
Myelomeningocele (MMC)
is the most common and most severe form of spina bifida. With myelomeningocele, a sac of fluid that holds part of the spinal cord and nerve tissue protrudes through an opening in the baby’s back. This damages the spinal cord and nerves through exposing them to the amniotic fluid. MMC can result in partial or complete paralysis of the body below the level of the spinal opening, an inability to walk, and/or bladder and bowel dysfunction.
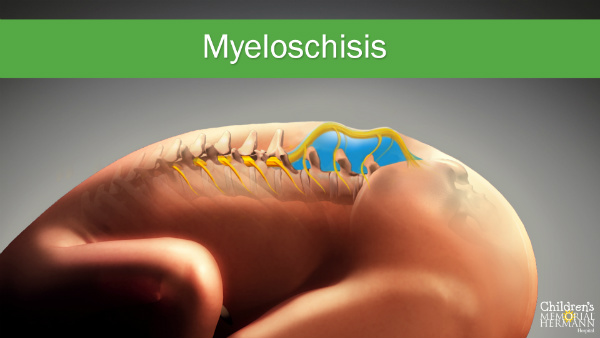
Myeloschisis
is a flat neural tube defect without a layer of skin covering the opening in the spine. With myeloschisis, the spinal cord and the surrounding nerve tissue are also exposed to the amniotic fluid. This form of spina bifida has similar risks and symptoms as myelomeningocele.
MMC and myeloschisis are both open neural tube defects, which are treatable by fetal repair surgery.
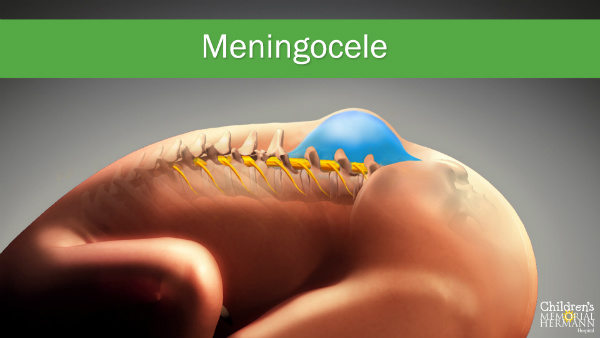 Meningocele
is a form of spina bifida where there is an outpouching or sac through an abnormal opening in the fetal spine, but the sac contains only spinal fluid. This abnormality does not contain any nerve tissues. Often the sac is covered by skin. Babies with meningocele may have few or no symptoms, while others may develop degrees of paralysis with bladder and bowel dysfunction.
Meningocele
is a form of spina bifida where there is an outpouching or sac through an abnormal opening in the fetal spine, but the sac contains only spinal fluid. This abnormality does not contain any nerve tissues. Often the sac is covered by skin. Babies with meningocele may have few or no symptoms, while others may develop degrees of paralysis with bladder and bowel dysfunction.
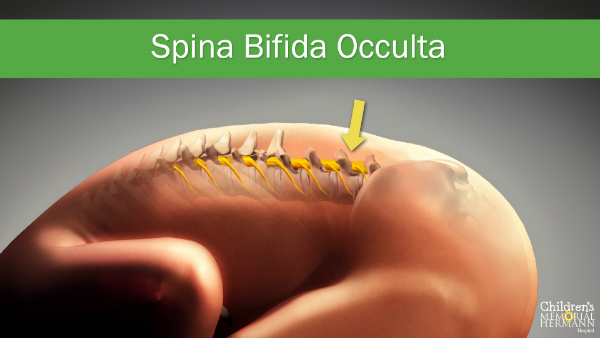 Spina bifida occulta,
or “hidden spina bifida,” is the mildest form of spina bifida, where a small gap in the spine occurs, but there is no opening or sac on the baby’s back. Often not discovered until late childhood or early adulthood, this condition usually does not cause any disabilities as the spinal cord and the nerves are typically normal.
Spina bifida occulta,
or “hidden spina bifida,” is the mildest form of spina bifida, where a small gap in the spine occurs, but there is no opening or sac on the baby’s back. Often not discovered until late childhood or early adulthood, this condition usually does not cause any disabilities as the spinal cord and the nerves are typically normal.
 Myelomeningocele (MMC)
is the most common and most severe form of spina bifida. With myelomeningocele, a sac of fluid that holds part of the spinal cord and nerve tissue protrudes through an opening in the baby’s back. This damages the spinal cord and nerves through exposing them to the amniotic fluid. MMC can result in partial or complete paralysis of the body below the level of the spinal opening, an inability to walk, and/or bladder and bowel dysfunction.
Myelomeningocele (MMC)
is the most common and most severe form of spina bifida. With myelomeningocele, a sac of fluid that holds part of the spinal cord and nerve tissue protrudes through an opening in the baby’s back. This damages the spinal cord and nerves through exposing them to the amniotic fluid. MMC can result in partial or complete paralysis of the body below the level of the spinal opening, an inability to walk, and/or bladder and bowel dysfunction.
 Meningocele
is a form of spina bifida where there is an outpouching or sac through an abnormal opening in the fetal spine, but the sac contains only spinal fluid. This abnormality does not contain any nerve tissues. Often the sac is covered by skin. Babies with meningocele may have few or no symptoms, while others may develop degrees of paralysis with bladder and bowel dysfunction.
Meningocele
is a form of spina bifida where there is an outpouching or sac through an abnormal opening in the fetal spine, but the sac contains only spinal fluid. This abnormality does not contain any nerve tissues. Often the sac is covered by skin. Babies with meningocele may have few or no symptoms, while others may develop degrees of paralysis with bladder and bowel dysfunction. Spina bifida occulta,
or “hidden spina bifida,” is the mildest form of spina bifida, where a small gap in the spine occurs, but there is no opening or sac on the baby’s back. Often not discovered until late childhood or early adulthood, this condition usually does not cause any disabilities as the spinal cord and the nerves are typically normal.
Spina bifida occulta,
or “hidden spina bifida,” is the mildest form of spina bifida, where a small gap in the spine occurs, but there is no opening or sac on the baby’s back. Often not discovered until late childhood or early adulthood, this condition usually does not cause any disabilities as the spinal cord and the nerves are typically normal.